To Change The Energy In A Substance, You Must Change What?
Chapter 5. Thermochemistry
5.1 Energy Basics
Learning Objectives
By the end of this section, you volition be able to:
- Ascertain energy, distinguish types of free energy, and depict the nature of energy changes that accompany chemical and physical changes
- Distinguish the related backdrop of oestrus, thermal energy, and temperature
- Ascertain and distinguish specific rut and estrus capacity, and describe the physical implications of both
- Perform calculations involving heat, specific heat, and temperature alter
Chemic changes and their accompanying changes in free energy are important parts of our everyday world (Figure 1). The macronutrients in nutrient (proteins, fats, and carbohydrates) undergo metabolic reactions that provide the energy to go along our bodies performance. We burn down a multifariousness of fuels (gasoline, natural gas, coal) to produce energy for transportation, heating, and the generation of electricity. Industrial chemical reactions apply enormous amounts of energy to produce raw materials (such as iron and aluminum). Free energy is so used to manufacture those raw materials into useful products, such equally cars, skyscrapers, and bridges.

Over xc% of the free energy nosotros use comes originally from the sun. Every day, the sun provides the earth with almost x,000 times the amount of free energy necessary to meet all of the world'south free energy needs for that day. Our challenge is to detect ways to catechumen and shop incoming solar free energy so that it tin exist used in reactions or chemic processes that are both convenient and nonpolluting. Plants and many bacteria capture solar energy through photosynthesis. We release the free energy stored in plants when we burn wood or plant products such as ethanol. Nosotros besides employ this energy to fuel our bodies by eating nutrient that comes directly from plants or from animals that got their energy by eating plants. Burning coal and petroleum too releases stored solar energy: These fuels are fossilized plant and animate being matter.
This chapter will introduce the bones ideas of an important surface area of science concerned with the amount of heat captivated or released during chemical and concrete changes—an area called thermochemistry. The concepts introduced in this affiliate are widely used in almost all scientific and technical fields. Nutrient scientists use them to determine the energy content of foods. Biologists study the energetics of living organisms, such as the metabolic combustion of sugar into carbon dioxide and water. The oil, gas, and transportation industries, renewable free energy providers, and many others endeavor to notice improve methods to produce energy for our commercial and personal needs. Engineers strive to improve energy efficiency, observe meliorate ways to estrus and absurd our homes, air-condition our food and drinks, and see the energy and cooling needs of computers and electronics, among other applications. Understanding thermochemical principles is essential for chemists, physicists, biologists, geologists, every type of engineer, and just about anyone who studies or does whatsoever kind of science.
Energy
Free energy can exist defined as the capacity to supply oestrus or practise piece of work. One type of work (westward) is the process of causing matter to motility confronting an opposing force. For example, we exercise piece of work when we inflate a bike tire—we move matter (the air in the pump) against the opposing forcefulness of the air already in the tire.
Like affair, energy comes in different types. One scheme classifies free energy into two types: potential energy, the energy an object has because of its relative position, composition, or condition, and kinetic energy, the energy that an object possesses considering of its motion. Water at the elevation of a waterfall or dam has potential energy because of its position; when information technology flows downwards through generators, it has kinetic free energy that can be used to do work and produce electricity in a hydroelectric constitute (Figure 2). A battery has potential energy because the chemicals inside it tin produce electricity that tin exercise piece of work.

Energy tin can be converted from i form into another, but all of the energy present earlier a alter occurs always exists in some form later on the change is completed. This observation is expressed in the law of conservation of energy: during a chemic or physical change, energy can be neither created nor destroyed, although information technology can be inverse in class. (This is besides i version of the offset police of thermodynamics, equally you volition learn later.)
When one substance is converted into some other, there is e'er an associated conversion of one form of free energy into some other. Heat is usually released or captivated, but sometimes the conversion involves low-cal, electric energy, or some other form of energy. For example, chemical energy (a blazon of potential free energy) is stored in the molecules that compose gasoline. When gasoline is combusted within the cylinders of a car's engine, the rapidly expanding gaseous products of this chemical reaction generate mechanical energy (a type of kinetic energy) when they movement the cylinders' pistons.
Co-ordinate to the constabulary of conservation of thing (seen in an earlier chapter), there is no detectable change in the total amount of thing during a chemical change. When chemical reactions occur, the energy changes are relatively modest and the mass changes are too small to mensurate, so the laws of conservation of affair and energy hold well. Nonetheless, in nuclear reactions, the energy changes are much larger (by factors of a million or and so), the mass changes are measurable, and matter-energy conversions are significant. This volition be examined in more detail in a later chapter on nuclear chemistry. To encompass both chemic and nuclear changes, nosotros combine these laws into one statement: The full quantity of matter and energy in the universe is fixed.
Thermal Energy, Temperature, and Heat
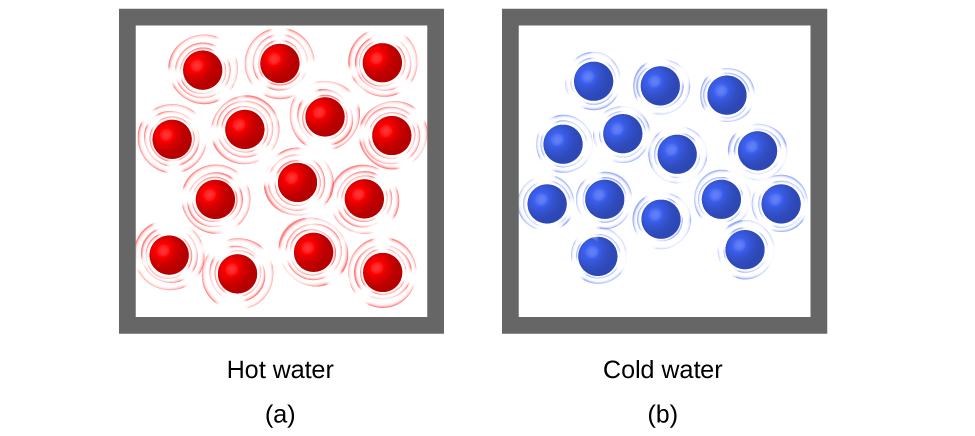
Thermal energy is kinetic energy associated with the random motion of atoms and molecules. Temperature is a quantitative measure out of "hot" or "cold." When the atoms and molecules in an object are moving or vibrating quickly, they accept a higher boilerplate kinetic free energy (KE), and we say that the object is "hot." When the atoms and molecules are moving slowly, they have lower KE, and nosotros say that the object is "common cold" (Figure iii). Assuming that no chemic reaction or stage change (such as melting or vaporizing) occurs, increasing the corporeality of thermal energy in a sample of matter will crusade its temperature to increase. And, assuming that no chemical reaction or stage change (such every bit condensation or freezing) occurs, decreasing the amount of thermal energy in a sample of thing will cause its temperature to subtract.

Click on this interactive simulation to view the furnishings of temperature on molecular motion.
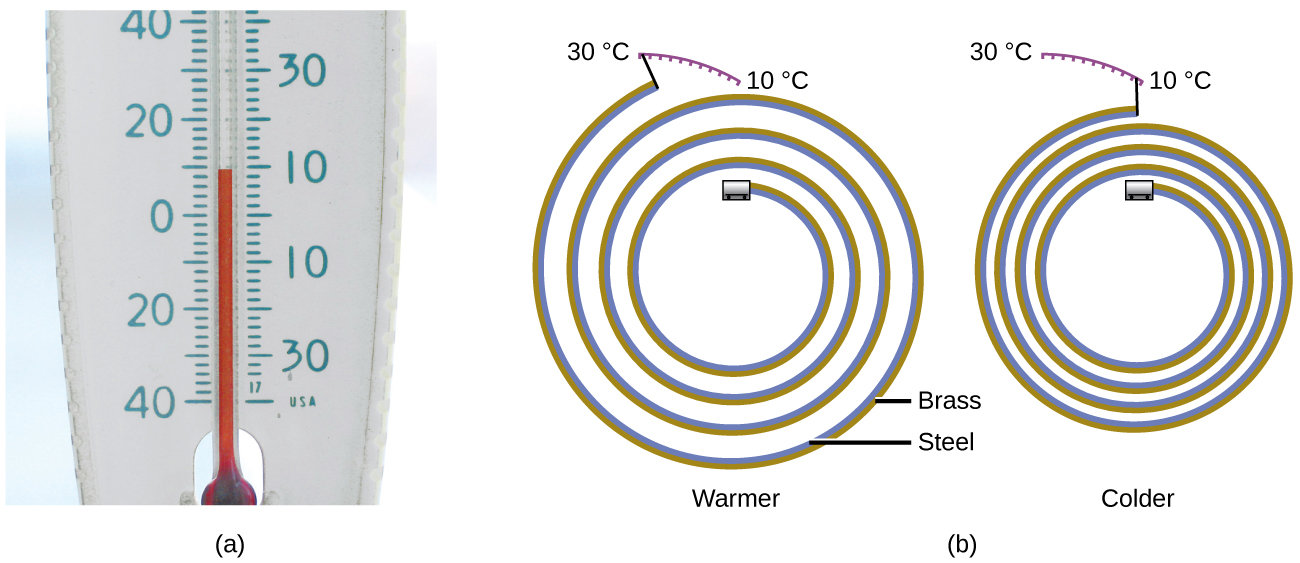
Almost substances aggrandize every bit their temperature increases and contract as their temperature decreases. This property can be used to measure temperature changes, as shown in Figure four. The performance of many thermometers depends on the expansion and contraction of substances in response to temperature changes.

The following demonstration allows one to view the effects of heating and cooling a coiled bimetallic strip.
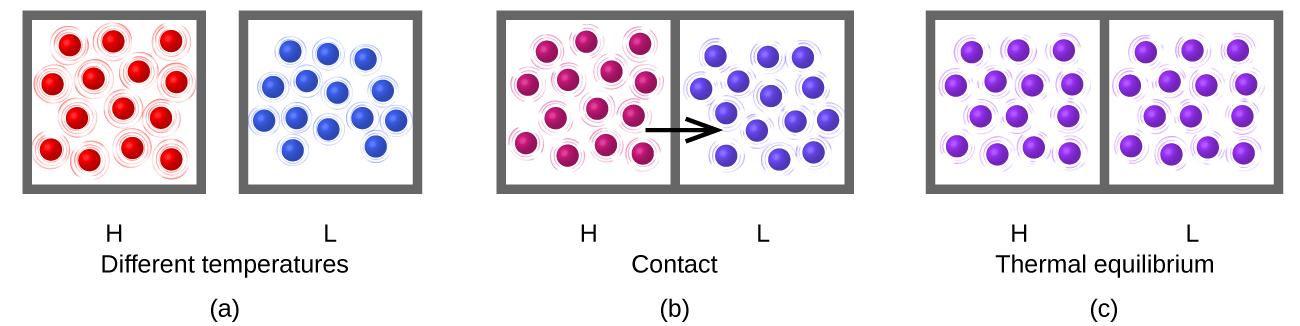
Heat (q) is the transfer of thermal energy between two bodies at unlike temperatures. Heat menstruum (a redundant term, but one commonly used) increases the thermal free energy of one torso and decreases the thermal energy of the other. Suppose we initially have a loftier temperature (and high thermal energy) substance (H) and a low temperature (and low thermal free energy) substance (L). The atoms and molecules in H have a higher average KE than those in L. If nosotros place substance H in contact with substance L, the thermal energy will flow spontaneously from substance H to substance L. The temperature of substance H will decrease, as will the average KE of its molecules; the temperature of substance L will increment, along with the average KE of its molecules. Heat flow will continue until the 2 substances are at the same temperature (Figure 5).

Click on the PhET simulation to explore energy forms and changes. Visit the Energy Systems tab to create combinations of free energy sources, transformation methods, and outputs. Click on Energy Symbols to visualize the transfer of free energy.
Thing undergoing chemic reactions and concrete changes can release or absorb heat. A modify that releases oestrus is chosen an exothermic process. For example, the combustion reaction that occurs when using an oxyacetylene torch is an exothermic process—this process also releases energy in the form of light equally evidenced by the torch's flame (Figure 6). A reaction or change that absorbs oestrus is an endothermic process. A cold pack used to care for muscle strains provides an example of an endothermic process. When the substances in the common cold pack (water and a common salt like ammonium nitrate) are brought together, the resulting procedure absorbs heat, leading to the awareness of common cold.

Historically, energy was measured in units of calories (cal). A calorie is the amount of energy required to enhance 1 gram of water by 1 degree C (1 kelvin). However, this quantity depends on the atmospheric pressure and the starting temperature of the water. The ease of measurement of free energy changes in calories has meant that the calorie is even so frequently used. The Calorie (with a capital C), or large calorie, ordinarily used in quantifying food energy content, is a kilocalorie. The SI unit of heat, piece of work, and energy is the joule. A joule (J) is defined equally the amount of energy used when a force of ane newton moves an object 1 meter. It is named in honor of the English physicist James Prescott Joule. I joule is equivalent to one kg yard2/due south2, which is also called 1 newton–meter. A kilojoule (kJ) is 1000 joules. To standardize its definition, ane calorie has been set to equal 4.184 joules.
We now innovate two concepts useful in describing rut flow and temperature change. The rut capacity (C) of a body of matter is the quantity of heat (q) information technology absorbs or releases when it experiences a temperature change (ΔT) of 1 caste Celsius (or equivalently, ane kelvin):
[latex]C = \frac{q}{\Delta T}[/latex]
Estrus chapters is determined by both the type and corporeality of substance that absorbs or releases estrus. It is therefore an extensive holding—its value is proportional to the amount of the substance.
For instance, consider the heat capacities of two cast iron frying pans. The rut capacity of the large pan is v times greater than that of the small pan because, although both are fabricated of the same material, the mass of the large pan is 5 times greater than the mass of the pocket-size pan. More mass means more than atoms are present in the larger pan, so it takes more energy to make all of those atoms vibrate faster. The heat capacity of the small cast iron frying pan is found by observing that it takes 18,150 J of energy to raise the temperature of the pan by 50.0 °C:
[latex]C_{\text{pocket-size pan}} = \frac{18,140 \;\text{J}}{fifty.0 \;^{\circ}\text{C}} = 363 \;\text{J/}^\circ\text{C}[/latex]
The larger cast atomic number 26 frying pan, while fabricated of the same substance, requires 90,700 J of energy to enhance its temperature by fifty.0 °C. The larger pan has a (proportionally) larger heat capacity because the larger amount of fabric requires a (proportionally) larger amount of energy to yield the same temperature change:
[latex]C_{\text{big pan}} = \frac{90,700 \;\text{J}}{fifty.0 \;^{\circ}\text{C}} = 1814 \;\text{J/}^\circ\text{C}[/latex]
The specific oestrus capacity (c) of a substance, commonly chosen its "specific oestrus," is the quantity of rut required to raise the temperature of ane gram of a substance by one caste Celsius (or ane kelvin):
[latex]C = \frac{q}{m\Delta T}[/latex]
Specific estrus capacity depends only on the kind of substance arresting or releasing heat. It is an intensive property—the blazon, only not the corporeality, of the substance is all that matters. For case, the pocket-size cast atomic number 26 frying pan has a mass of 808 g. The specific heat of iron (the material used to make the pan) is therefore:
[latex]c_{\text{iron}} = \frac{eighteen,140 \;\text{J}}{(808 \;\text{g})(50.0 \;^{\circ}\text{C})} = 0.449 \;\text{J/g} \;^\circ\text{C}[/latex]
The large frying pan has a mass of 4040 m. Using the data for this pan, we can also calculate the specific heat of iron:
[latex]c_{\text{atomic number 26}} = \frac{ninety,700 \;\text{J}}{(4040 \;\text{k})(50.0 \;^{\circ}\text{C})} = 0.449 \;\text{J/g} \;^\circ\text{C}[/latex]
Although the large pan is more massive than the small pan, since both are made of the aforementioned cloth, they both yield the same value for specific heat (for the cloth of construction, iron). Notation that specific heat is measured in units of free energy per temperature per mass and is an intensive belongings, being derived from a ratio of 2 extensive properties (estrus and mass). The molar oestrus chapters, besides an intensive property, is the oestrus capacity per mole of a detail substance and has units of J/mol °C (Figure 7).

Liquid h2o has a relatively high specific oestrus (about 4.ii J/thousand °C); most metals have much lower specific heats (usually less than 1 J/thou °C). The specific heat of a substance varies somewhat with temperature. However, this variation is commonly modest plenty that we volition treat specific heat equally constant over the range of temperatures that volition be considered in this affiliate. Specific heats of some common substances are listed in Tabular array one.
| Substance | Symbol (state) | Specific Oestrus (J/grand °C) |
|---|---|---|
| helium | He(g) | 5.193 |
| water | HtwoO(l) | 4.184 |
| ethanol | C2HviO(l) | 2.376 |
| ice | HtwoO(s) | 2.093 (at −ten °C) |
| water vapor | H2O(k) | 1.864 |
| nitrogen | N2(grand) | 1.040 |
| air | ||
| oxygen | O2(g) | 0.918 |
| aluminum | Al(s) | 0.897 |
| carbon dioxide | CO2(g) | 0.853 |
| argon | Ar(thousand) | 0.522 |
| iron | Fe(southward) | 0.449 |
| copper | Cu(southward) | 0.385 |
| lead | Atomic number 82(s) | 0.130 |
| gold | Au(s) | 0.129 |
| silicon | Si(s) | 0.712 |
| Table 1. Specific Heats of Common Substances at 25 °C and 1 bar | ||
If we know the mass of a substance and its specific heat, we tin can decide the amount of rut, q, inbound or leaving the substance by measuring the temperature change earlier and subsequently the rut is gained or lost:
[latex]\begin{assortment} {r @{{}={}} l} q & \text{(specific oestrus)} \times \text{(mass of substance)} \times \text{(temperature alter)} \\[1em] q & c \times m \times \Delta T =c \times m \times (T_{\text{concluding}} - T_{\text{initial}}) \end{assortment}[/latex]
In this equation, c is the specific heat of the substance, thou is its mass, and ΔT (which is read "delta T") is the temperature modify, T last − T initial. If a substance gains thermal energy, its temperature increases, its final temperature is higher than its initial temperature, T final − T initial has a positive value, and the value of q is positive. If a substance loses thermal energy, its temperature decreases, the final temperature is lower than the initial temperature, T final − T initial has a negative value, and the value of q is negative.
Case one
Measuring Heat
A flask containing viii.0 × x2 g of water is heated, and the temperature of the h2o increases from 21 °C to 85 °C. How much rut did the h2o absorb?
Solution
To answer this question, consider these factors:
- the specific heat of the substance beingness heated (in this case, h2o)
- the amount of substance being heated (in this case, 800 yard)
- the magnitude of the temperature modify (in this case, from 21 °C to 85 °C).
The specific heat of water is four.184 J/g °C, then to estrus i g of h2o past ane °C requires four.184 J. We note that since 4.184 J is required to heat one g of water past 1 °C, we will need 800 times as much to rut 800 thousand of water by 1 °C. Finally, we observe that since four.184 J are required to heat one m of water by i °C, we will need 64 times as much to heat it by 64 °C (that is, from 21 °C to 85 °C).
This tin be summarized using the equation:
[latex]\begin{array} {r@ {{}={}} l} q & c \times m \times \Delta T = c \times m \times (T_{\text{final}} - T_{\text{initial}}) \\[1em] & (4.184 \;\text{J/}\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g} \;^\circ\text{C} \times (800 \;\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{g}) \times (85 - 20) \;^\circ\text{C} \\[1em] & (4.184 \;\text{J/}\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{chiliad} \;^\circ\dominion[0.5ex]{0.75em}{0.1ex}\hspace{-0.75em}\text{C} \times (800 \;\rule[0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}\text{thou}) \times (65) \;^\circ\rule[0.5ex]{0.75em}{0.1ex}\hspace{-0.75em}\text{C} \\[1em] & 210,000 \;\text{J} (= 210 \;\text{kJ}) \cease{array}[/latex]
Because the temperature increased, the water captivated rut and q is positive.
Bank check Your Learning
How much heat, in joules, must exist added to a 5.00 × 10two-thousand iron skillet to increase its temperature from 25 °C to 250 °C? The specific oestrus of iron is 0.451 J/grand °C.
Note that the relationship between estrus, specific heat, mass, and temperature change tin exist used to decide any of these quantities (non just estrus) if the other three are known or can be deduced.
Example two
Determining Other Quantities
A piece of unknown metal weighs 348 g. When the metal piece absorbs half dozen.64 kJ of heat, its temperature increases from 22.iv °C to 43.6 °C. Determine the specific heat of this metal (which might provide a clue to its identity).
Solution
Since mass, heat, and temperature change are known for this metal, nosotros can determine its specific heat using the relationship:
[latex]q = c \times yard \times \Delta T = c \times m \times (T_{\text{final}} - T_{\text{initial}})[/latex]
Substituting the known values:
[latex]6640 \;\text{J} = c \times (348 \;\text{g}) \times (43.6 - 22.iv) \;^\circ\text{C}[/latex]
Solving:
[latex]c = \frac{6640 \;\text{J}}{(348 \;\text{chiliad}) \times (21.ii \;^\circ\text{C})} = 0.900 \;\text{J/k} \;^\circ\text{C}[/latex]
Comparing this value with the values in Table 1, this value matches the specific heat of aluminum, which suggests that the unknown metal may be aluminum.
Check Your Learning
A piece of unknown metal weighs 217 m. When the metal slice absorbs 1.43 kJ of estrus, its temperature increases from 24.5 °C to 39.ane °C. Determine the specific oestrus of this metal, and predict its identity.
Reply:
c = 0.45 J/g °C; the metal is likely to be iron
Note: Solar Thermal Energy Power Plants
The sunlight that reaches the globe contains thousands of times more free energy than we before long capture. Solar thermal systems provide 1 possible solution to the trouble of converting energy from the dominicus into free energy we can employ. Large-scale solar thermal plants have dissimilar pattern specifics, but all concentrate sunlight to heat some substance; the heat "stored" in that substance is and so converted into electricity.
The Solana Generating Station in Arizona's Sonora Desert produces 280 megawatts of electrical power. It uses parabolic mirrors that focus sunlight on pipes filled with a heat transfer fluid (HTF) (Figure 8). The HTF then does 2 things: It turns water into steam, which spins turbines, which in turn produces electricity, and it melts and heats a mixture of salts, which functions as a thermal energy storage system. After the dominicus goes downwardly, the molten salt mixture can then release enough of its stored heat to produce steam to run the turbines for half-dozen hours. Molten salts are used considering they possess a number of benign properties, including high heat capacities and thermal conductivities.
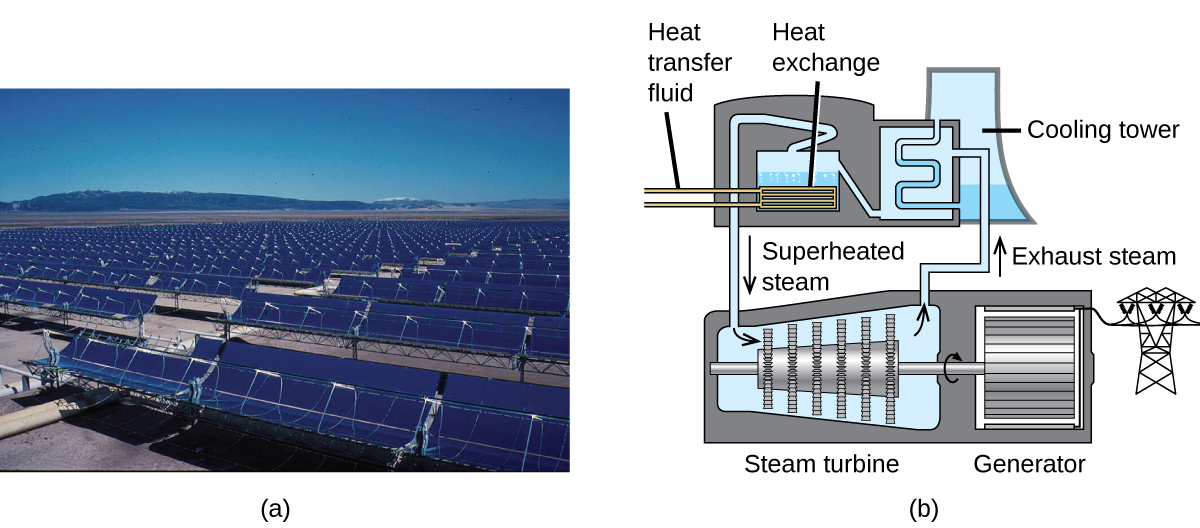
The 377-megawatt Ivanpah Solar Generating Organization, located in the Mojave Desert in California, is the largest solar thermal power institute in the world (Figure 9). Its 170,000 mirrors focus huge amounts of sunlight on three water-filled towers, producing steam at over 538 °C that drives electricity-producing turbines. It produces enough energy to ability 140,000 homes. Water is used as the working fluid considering of its large heat capacity and heat of vaporization.

Key Concepts and Summary
Free energy is the chapters to do work (applying a strength to move matter). Kinetic energy (KE) is the energy of motion; potential energy is energy due to relative position, composition, or condition. When free energy is converted from one class into another, energy is neither created nor destroyed (law of conservation of energy or get-go constabulary of thermodynamics).
Matter has thermal energy due to the KE of its molecules and temperature that corresponds to the average KE of its molecules. Heat is energy that is transferred betwixt objects at different temperatures; it flows from a high to a low temperature. Chemical and physical processes can absorb rut (endothermic) or release estrus (exothermic). The SI unit of free energy, heat, and work is the joule (J).
Specific heat and estrus capacity are measures of the free energy needed to alter the temperature of a substance or object. The amount of heat absorbed or released by a substance depends directly on the blazon of substance, its mass, and the temperature change it undergoes.
Key Equations
- [latex]q = c \times m \times \Delta T = c \times m \times (T_{\text{final}} - T_{\text{initial}})[/latex]
Chemistry End of Chapter Exercises
- A burning friction match and a bonfire may have the same temperature, nevertheless y'all would not sit down effectually a burning match on a autumn evening to stay warm. Why non?
- Prepare a table identifying several energy transitions that accept place during the typical functioning of an automobile.
- Explain the divergence between estrus capacity and specific oestrus of a substance.
- Calculate the heat chapters, in joules and in calories per degree, of the post-obit:
(a) 28.4 g of h2o
(b) 1.00 oz of lead
- Calculate the estrus capacity, in joules and in calories per degree, of the post-obit:
(a) 45.8 g of nitrogen gas
(b) 1.00 pound of aluminum metallic
- How much heat, in joules and in calories, must exist added to a 75.0–1000 iron cake with a specific heat of 0.449 J/thousand °C to increase its temperature from 25 °C to its melting temperature of 1535 °C?
- How much rut, in joules and in calories, is required to heat a 28.4-thou (1-oz) water ice cube from −23.0 °C to −ane.0 °C?
- How much would the temperature of 275 g of water increase if 36.5 kJ of oestrus were added?
- If fourteen.v kJ of heat were added to 485 g of liquid water, how much would its temperature increase?
- A piece of unknown substance weighs 44.7 chiliad and requires 2110 J to increase its temperature from 23.2 °C to 89.6 °C.
(a) What is the specific heat of the substance?
(b) If it is one of the substances found in Table 1, what is its likely identity?
- A piece of unknown solid substance weighs 437.2 one thousand, and requires 8460 J to increase its temperature from xix.iii °C to 68.9 °C.
(a) What is the specific heat of the substance?
(b) If information technology is one of the substances plant in Table one, what is its likely identity?
- An aluminum kettle weighs 1.05 kg.
(a) What is the heat chapters of the kettle?
(b) How much heat is required to increase the temperature of this kettle from 23.0 °C to 99.0 °C?
(c) How much heat is required to heat this kettle from 23.0 °C to 99.0 °C if it contains 1.25 L of water (density of 0.997 g/mL and a specific heat of four.184 J/thousand °C)?
- Most people find waterbeds uncomfortable unless the water temperature is maintained at nigh 85 °F. Unless it is heated, a waterbed that contains 892 L of water cools from 85 °F to 72 °F in 24 hours. Estimate the amount of electrical energy required over 24 hours, in kWh, to keep the bed from cooling. Note that 1 kilowatt-hour (kWh) = iii.half-dozen × 10vi J, and assume that the density of water is 1.0 1000/mL (independent of temperature). What other assumptions did you make? How did they affect your calculated result (i.east., were they probable to yield "positive" or "negative" errors)?
Glossary
- calorie (cal)
- unit of heat or other free energy; the corporeality of energy required to heighten 1 gram of h2o past i degree Celsius; 1 cal is defined as iv.184 J
- endothermic process
- chemical reaction or physical change that absorbs heat
- free energy
- chapters to supply heat or do piece of work
- exothermic process
- chemical reaction or physical change that releases estrus
- estrus (q)
- transfer of thermal energy between 2 bodies
- estrus capacity (C)
- extensive property of a body of affair that represents the quantity of rut required to increase its temperature by i caste Celsius (or 1 kelvin)
- joule (J)
- SI unit of energy; i joule is the kinetic energy of an object with a mass of two kilograms moving with a velocity of one meter per second, i J = i kg 10002/s and iv.184 J = 1 cal
- kinetic energy
- energy of a moving trunk, in joules, equal to [latex]\frac{1}{two}mv^two[/latex] (where m = mass and v = velocity)
- potential free energy
- energy of a particle or system of particles derived from relative position, limerick, or condition
- specific heat capacity (c)
- intensive property of a substance that represents the quantity of heat required to heighten the temperature of ane gram of the substance by 1 degree Celsius (or 1 kelvin)
- temperature
- intensive property of affair that is a quantitative measure of "hotness" and "coldness"
- thermal energy
- kinetic free energy associated with the random motion of atoms and molecules
- thermochemistry
- report of measuring the amount of heat absorbed or released during a chemical reaction or a physical modify
- piece of work (w)
- free energy transfer due to changes in external, macroscopic variables such as pressure and volume; or causing matter to move against an opposing force
Solutions
Answers to Chemistry End of Chapter Exercises
1. The temperature of ane gram of called-for forest is approximately the same for both a match and a bonfire. This is an intensive holding and depends on the cloth (wood). Withal, the overall corporeality of produced heat depends on the amount of material; this is an all-encompassing belongings. The amount of wood in a bonfire is much greater than that in a match; the total amount of produced rut is too much greater, which is why we can sit around a bonfire to stay warm, but a match would not provide enough heat to keep us from getting cold.
3. Heat capacity refers to the heat required to raise the temperature of the mass of the substance one caste; specific rut refers to the heat required to raise the temperature of 1 gram of the substance 1 degree. Thus, estrus chapters is an extensive holding, and specific estrus is an intensive one.
5. (a) 47.six J/°C; 11.38 cal °C−1; (b) 407 J/°C; 97.three cal °C−1
seven. 1310; 313 cal
9. 7.15 °C
11. (a) 0.390 J/g °C; (b) Copper is a likely candidate.
xiii. We presume that the density of h2o is 1.0 chiliad/cmthree(1 k/mL) and that it takes as much energy to keep the water at 85 °F as to heat it from 72 °F to 85 °F. We as well assume that only the water is going to exist heated. Energy required = 7.47 kWh
Source: https://opentextbc.ca/chemistry/chapter/5-1-energy-basics/
Posted by: suttonthereatend.blogspot.com


0 Response to "To Change The Energy In A Substance, You Must Change What?"
Post a Comment